USP Monograph
The United States Pharmacopeia – National Formulary (USP-NF) includes over 5000 quality standards for medicines, both chemical and biologic; active pharmaceutical ingredients (APIs); and excipients (inactive ingredients). A monograph is a written document that reflects the quality attributes of medicines approved by the U.S. Food and Drug Administration (US FDA).
Original 3d concepts for the USP quality standards program.
Disclaimer: This is not a USP product. USP does not create products or labels for products.

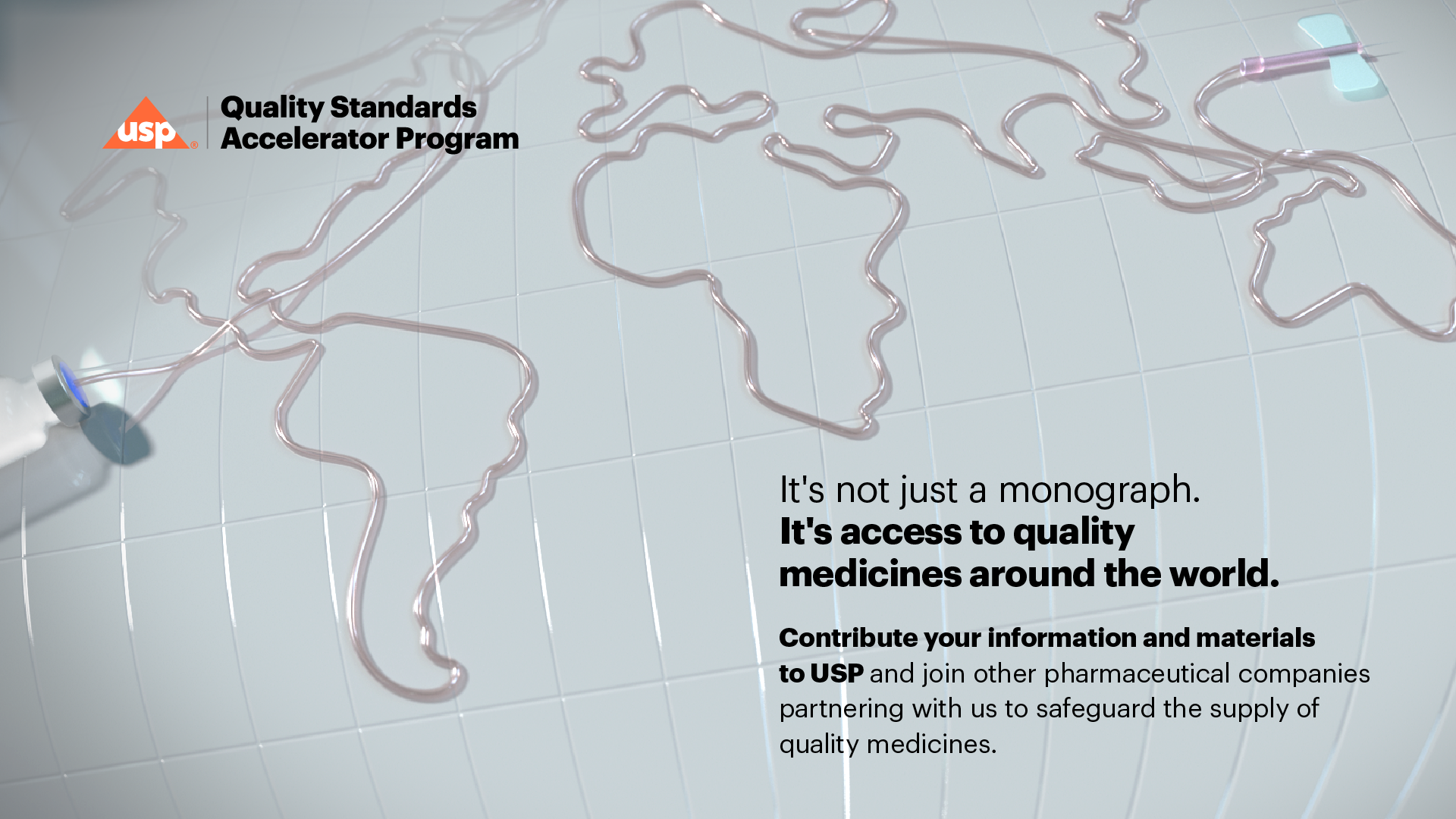




Bespoke concept frames